-
United States -
United Kingdom -
India -
France -
Deutschland -
Italia -
日本 -
대한민국 -
中国 -
台灣
-
Ansys si impegna a fare in modo che gli studenti di oggi abbiano successo, fornendogli il software gratuito di simulazione ingegneristica.
-
Ansys si impegna a fare in modo che gli studenti di oggi abbiano successo, fornendogli il software gratuito di simulazione ingegneristica.
-
Ansys si impegna a fare in modo che gli studenti di oggi abbiano successo, fornendogli il software gratuito di simulazione ingegneristica.
-
Contattaci -
Opportunità di lavoro -
Studenti e Accademici -
Per Stati Uniti e Canada
+1 844.462.6797
ANSYS的博客
February 17, 2022
Ansys Minerva Streamlines Credibility Assessment for Healthcare in Silico Testing
Documenting and implementing the extensive credibility requirements involved in simulation-based medical device testing, also referred to as in silico testing, is time consuming and may lack consistency, traceability, and security within an organization. To address these needs, Ansys has developed a template-based, end-to-end workflow within ourAnsys Minervasimulation process and data management (SPDM) platform. This tool guides users through the medical device industry’s most rigorous standards surrounding computational modeling and simulation (CM&S) as outlined by the American Society of Mechanical Engineers (ASME) V&V 40 standard on “Assessing Credibility of Computational Modeling through Verification and Validation: Application to Medical Devices”.
First Things First
CM&S is a popular approach that is used by the medical device and pharmaceutical industries throughout the process and product lifecycle, from ideation to post-market evaluation. It is beneficial in complementing traditional practices, such as bench testing, animal testing, and clinical trials, to establish device safety and effectiveness. But the healthcare industry has long struggled with establishing the model credibility needed to support regulatory decision-making.
An increasing number of in silico studies have revealed advantages over other forms of device evaluation. Some key benefits include the ability to conduct parametric, statistical, and even patient-specific analyses of device performance in well-controlled settings and in earlier stages of product development without any risk for patients. But model credibility must be established to qualify it as acceptable digital evidence for the regulatory approval process.
To address this issue, ASME formed a subcommittee on the verification and validation of computational models of medical devices (ASME V&V 40 subcommittee) in 2011 and published its first standard in 2018.
Today, ASME V&V 40 is recognized by the U.S. Food and Drug Administration (FDA) and used as a reference in other regions around the world. The standard guides users through the numerous steps for establishing and assessing the verification, validation, and uncertainty quantification requirements for computational models for a specific context of use (COU).
Minerva Makes It Easy
Ansys Minerva is an SPDM software platform that provides best-in-class data management, hybrid deployment, and integration with industry-leading tools. Using the customization capabilities of Minerva, Ansys engineers recently developed a Minerva template that simplifies navigating and tracking all aspects of model development by implementing the V&V 40 standard procedure in a step-by-step, user-friendly manner.
From identifying credibility requirements to defining simulation work requests and communicating with internal stakeholders, the Ansys Minerva solution tracks and manages the entire digital thread for computational modeling data throughout the product development process.
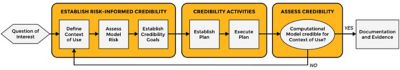
Figure 1. The risk-informed credibility assessment framework, as defined by the ASME V&V 40 standard
See for Yourself
Whether you are an engineer or healthcare industry professional using or managing CM&S, responsible for quality assurance, or a regulatory affairs professional, you will benefit from discovering how the new template in Ansys Minerva can assist your credibility data management.
Take a deeper dive byrequesting a demo of the Ansys Minerva platform.
Scopri cosa può fare Ansys per te
Scopri cosa può fare Ansys per te
Contattaci subito
Grazie per averci contattato!
We’re here to answer your questions and look forward to speaking with you. A member of our Ansys sales team will contact you shortly.













